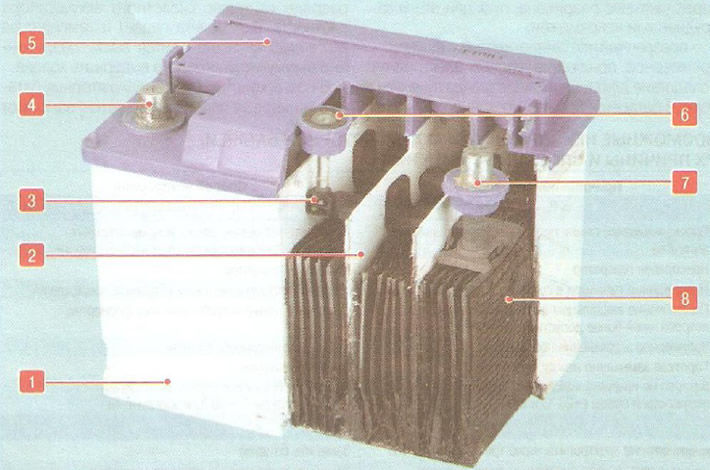
Pic. 10.3. Battery device: 1 - monoblock (frame); 2 - partition of elements; 3 - density indicator float; 4 - terminal «minus»; 5 - common cover; 6 - electrolyte density indicator; 7 - terminal «plus»; 8 - battery cell
A modern car is saturated with various consumers of electricity, electronic devices, starting with a CD player and ending with blocks of electronic engine control systems, a gearbox, an anti-lock brake system. airbags, etc. In the wet off-season, and especially in winter, all automotive electrics and electronics, and especially the car battery, are tested for endurance.
As practice shows, if there are problems with starting the engine in the cold season and to solve them, you constantly have to recharge the battery, provided that the generator is in good condition and the generator belt tension is normal, and the battery life exceeds 3 years, it is quite reasonable to raise the question of buying a new battery.
Modern batteries are usually of two types:
- maintenance-free during the entire service life;
- low-maintenance, requiring topping up with distilled water once or twice a year.
It is advisable to choose for your car, taking into account the recommendations of the manufacturer, a maintenance-free battery from a wide range of car batteries from various manufacturers on the automotive aftermarket.
Table 10.3. Dependence of battery capacity on electrolyte temperature
| Electrolyte temperature,°C | Battery capacity, % |
| -10 | 80 |
| -20 | 66 |
| -30 | 50 |
| -40 | 32 |
It must be remembered that at low temperatures, due to an increase in the viscosity of engine oil and a deterioration in the ignition conditions of the fuel, the power consumed by the starter when starting the engine increases by two to three times. The starting time of a cold engine in comparison with a warm one in some cases increases by 10-20 times. Thus, in winter, at low air temperatures, increased requirements are placed on the starter characteristics of the battery, i.e. to her ability for a short time (10 s according to GOST) to give the required current necessary for the operation of the starter with the nominal speed of its armature in the cold season (-18°C according to GOST).
In table. 10.3 shows the dependence of the battery capacity on the electrolyte temperature. Battery capacity is shown as a percentage of the battery capacity at 25°C.
Battery cells are located in a polypropylene monoblock (corps) 1 (pic. 10.3) and closed with a common cover 5, inseparably connected to the monoblock. The two vents on the sides of the battery at the top allow the small amount of gas generated in the battery to escape.
An electrolyte density indicator 6 can be mounted in the battery cover, the readings of which take into account the temperature of the battery. There are three options for the indicator readings:
- green dot - battery is charged;
- dark indicator without a green dot - the battery is partially discharged, starting the engine is difficult or impossible;
- transparent or light yellow indicator - an excessive decrease in the electrolyte level due to a long recharge of the battery or its natural wear.
A lead starter maintenance-free battery with a capacity of 55 Ah is installed on the car. Rechargeable batteries of the specified capacity are produced by many manufacturers, the characteristics of these batteries are similar.
Note: The principle of operation of lead-acid batteries is based on the electrochemical reactions of lead and lead dioxide in a sulfuric acid environment. During the discharge, lead dioxide is reduced at the cathode and lead is oxidized at the anode. When charging, reverse reactions occur, to which, at the end of the charge, the water electrolysis reaction is added, accompanied by the release of oxygen at the positive electrode and hydrogen at the negative.
The lead-acid battery cell consists of positive and negative electrodes, separators (dividing grids) and electrolyte. The positive electrodes are a lead grid in which the active substance is lead peroxide (PbO 2). The negative electrodes are also a lead grid with spongy lead as the active substance. In practice, 1-2% antimony is added to the lead gratings to increase the mechanical strength. Currently, calcium salts are used as an alloying component in both plates or only in positive ones (hybrid technology). The electrodes are immersed in an electrolyte consisting of an aqueous solution of sulfuric acid (H 2 SO 4). The highest conductivity of this solution at room temperature (which means the smallest internal resistance and the smallest internal losses) achieved at its density of 1.26 g/cm3. However, in practice, in regions with a cold climate, higher concentrations of sulfuric acid are also used - up to 1.29-1.31 g / cm3. This is done because when a lead-acid battery is discharged, the density of the electrolyte drops and its freezing point becomes higher, a discharged battery may not withstand the cold.
In new battery designs, lead plates (gratings) is replaced with foamed carbon coated with a thin lead film, and the liquid electrolyte is gelled with silica gel to a pasty state. By using less lead and distributing it over a large area, the battery is not only smaller and lighter, but much more efficient; in addition to greater efficiency, it charges much faster than previous generation batteries.
Notes: An electrolyte density indicator may be mounted in the battery cover, the readings of which take into account the temperature of the battery. There are three options for the indicator readings:
- green dot - battery is charged;
- dark indicator without a green dot - the battery is partially discharged, starting the engine is difficult or impossible;
- transparent or light yellow indicator - an excessive decrease in the electrolyte level due to a long recharge of the battery or its natural wear.
Instead of a regular maintenance-free battery, you can install any battery of other manufacturers that is similar in capacity and mounting dimensions. In this case, use and maintain the battery in accordance with the instructions supplied with it.
Warnings: Batteries of the same model can be made in two versions with different connection polarity (conclusions «plus» And «minus» batteries of different variants are located oppositely). Purchase a battery of the same polarity as that installed on the car, since a battery of a different polarity cannot be connected to the on-board network due to the insufficient length of the wires and the mismatch in the size of their tips. In addition, some manufacturers produce batteries with reduced terminal sizes (another standard), which also cannot be connected to the on-board network of your car.
When working with metal tools, do not short-circuit the battery.
When charging the battery, an explosive gas mixture is released, therefore, during charging and servicing the battery, it is forbidden to smoke and use open fire. Charge the battery in a well ventilated area.
In case of accidental splashes of electrolyte on the skin or eyes, immediately, before receiving medical attention, rinse the affected area with plenty of water and then with a 2% solution of baking soda (0.5 teaspoon per glass of water).
Always wash your hands with soap and water after any work on the battery.
Note: Instead of a regular maintenance-free battery, you can install any battery similar in voltage, capacity, mounting dimensions and connection polarity. In this case, use and maintain the battery in accordance with the instructions supplied with it.
